Organometallic compounds
The elements create compounds R2M and RMX, in which R is an alkyl or aryl group and X a halide. M-C bond strengths are in the order Zn>Cd>Hg but yet the mercury compounds are the very easily formed; for instance, from
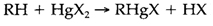
In the direction of air or water the Hg compounds are also the least reactive, partly due to the competing Hg-O bond is so much weaker than with Zn or Cd. They are helpful for preparing organometallic compounds of other elements. Water-soluble ions could be acquired, like [CH3Hg]+, which has been employed like a prototype 'soft' acid in the hard and soft acid and base (HSAB) classification. All organomercury compounds are very toxic, like they go through cell membranes much more simply than inorganic forms.