MII solid compounds
Only the fluorides comprise structures and properties supposed for ionic compounds with cations of the suitable size (ZnF2 rutile, the anothers fluorite). The characteristic coordination numbers are 4 for Zn, 4 or 6 for Cd, and two or four for Hg in other compounds.
Zn and Cd halides (except fluorides) are based on close-packed lattices of halide ions, with Zn taking place tetrahedral holes and Cd octahedral ones. The Zn compounds are best considered as polymeric, where those of Cd are prototypes of the significant CdCl2 and CdI2 layer structures. In water both sets of compounds are soluble, but solutions of Cd halides consist of a variety of complex ions [CdXn] in equilibrium. Hg halides consist of varying coordination, with two close neighbors within HgCl2 making this compound necessarily molecular, another being more polymeric. Solubility in water is low but increases noticeably with increase in temperature, providing undissociated HgX2 molecules.
Between the oxides and sulfides, only CdO accepts the octahedral rocksalt structure found with group 2 elements, even though the solid is generally very deficient in oxygen and the electrons not employed in bonding give rise to metallic properties. ZnS and ZnO are prototypes of the tetrahedrally coordinated zinc and wurtzite blende (or sphalerite) structures; actually, ZnS can adopt either structure, like can CdS and CdSe. HgO and HgS contain chain structures with linear two-coordination of Hg. Several of these compounds are colored and depict electronic properties characteristic of small bandgaps and nonstoichiometry.
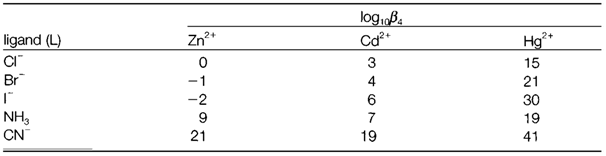
Table 1. Overall equilibrium constants (log10β4) for the formation of some [ML4] complexes