Lower oxidation states
The +1 oxidation state is quite stable for mercury, and always involves the dimeric [Hg-Hg]2+ ion. Proof for this comes from solid-state structures and in solution from several sources:
- HgI species are diamagnetic where Hg+ would have an unpaired electron;
- Raman spectra of solutions depict a band from the Hg-Hg stretching vibration identical to that seen in solids;
- Equilibrium studies (example through electrochemistry) are consistent with
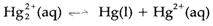
with an equilibrium constant [Hg2+]/[ Hg2+2]at 25°C; the equilibrium expression including Hg+ would have a dissimilar form.
In aqueous solution Uncomplexed Hg2+2 is marginally stable, but the disproportionation equilibrium can be upset through any ligand for which the HgII compound is more stable. So addition of sulfide, cyanide and several other ligands causes disproportionation. In solid compounds the Hg2+2 ion all the time has two ligands strongly bonded. For instance, Hg2Cl2 has linear Cl-Hg-Hg-Cl molecules and salts with noncomplexing anions like nitrate consist the hydrated ion [H2O-Hg-Hg-H2O]2+.
Oxidation of Hg with AsF5 gives species consisting of linear Hg2+3and Hg2+4ions, culminating in a metallic compound Hg0.33AsF6, that consists of linear chains of mercury atoms. Zn and Cd analogs of Hg2+2are much less stable, mainly due to the larger lattice energies acquired with the smaller M2+ ions tend to force disproportionation. Zn2+2 and Cd2+2 can both be recognized spectroscopically while the elements react with melts of the subsequent chloride. Adding AlCl3 provides the solid compound [Cd2+2] [AlCl4-]2but no solid zinc (I) compounds have been ready.