Cells:
Several kinds of cells have been designed and are in use for the measurement of conductance of a solution. Those are made of Pyrex glass fitted along with electrodes of platinum or gold. For overcome the imperfections within the current and the other effects at the electrodes, these are coated within a layer of finely separated platinum black. This is achieved through electrolyzing a 3 percent solution of chloroplatinic acid containing a little of lead acetate. The distance among the electrodes is determined through the conductance of the solution to be measured. To highly conducting solution, the electrodes are widely spaced whereas for low conducting solutions the electrodes are mounted near each other. A cell suitable for conductometric titration is depicted in Figure (a, b and c); the electrodes are firmly fixed in the Perspex lid that is given along with opening for the stirrer and the jet of the burette. A magnetic stirrer could be used in place of mechanical stirrer.
For most reasons a special cell is not needed and good results are obtained through clamping a commercially available dip cell [shown diagrammatically in Figure (b)] inside a beaker that is placed on a magnetic stirrer. With this arrangement, the dipping cell should be lifted clear of the solution after each addition from the burette to ensure that the liquid between the electrodes becomes thoroughly mixed. Because absolute conductivity values are not needed it is not essential to know the cell constant.
For spot checking on a procedure tank or stream, a dip-type of conductivity cell is used. Within a few titrations on open beaker with fixed electrodes is sufficient. Therefore, for fairly dilute solutions an open beaker would not be satisfactory since atmospheric CO2 may alter the conductance.
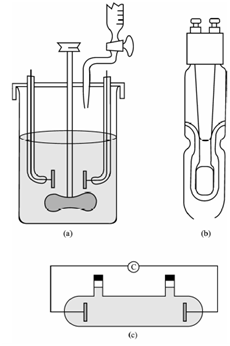
Figure: Typical designs of conductivity cells