Typical Ion Exchanger:
In sequence of decreasing strength, the associative affinities among a cation resin and several cations are as follows.
Ba+2 > Sr+2 > Ca+2 > Co+2 > Ni+2 > Cu+2 > Mg+2 > Be+2
Ag+ > Cs+ > Rb+ > K+ ≈NH+ > Na+ > H+ > Li+
As same, the associative affinities among an anion resin and several anions are as follows.
SO4-2 > I- >NO3- > Br- >HSO3- > Cl- > OH- > HCO3- >F-
The physical arrangement of one category of ion exchange vessel for purifying water is display in Figure. The ion exchange resin is holed in a vessel along with a volume of various cubic feet. Retention components at the top and bottom consist of screens, slotted cylinders, and the another appropriate devices along with openings smaller than the resin beads to avoid the resin from escaping from the vessel. A resin bed is a uniform combination of cation and anion resins in a volume ratio of 2 categories cation resin to 3 categories anion resin. This arrangement is known as a mixed-bed resin, as opposed to an arrangement of cation and anion resins in discrete layers or divided vessels. The use of various volumes of the two categories of resins is because of the difference within exchange capacity among cation and anion resins. Replace capacity is the amount of impurity in which a provided amount of resin is capable of erasing, and it has units of equivalents/ml, moles/ml, or moles/gm. The anion resin is less dense than the cation resin; thus, it has a smaller exchange capacity, or a larger volume is required for anion resins than for the cation resins to acquire equivalent total exchange capabilities.
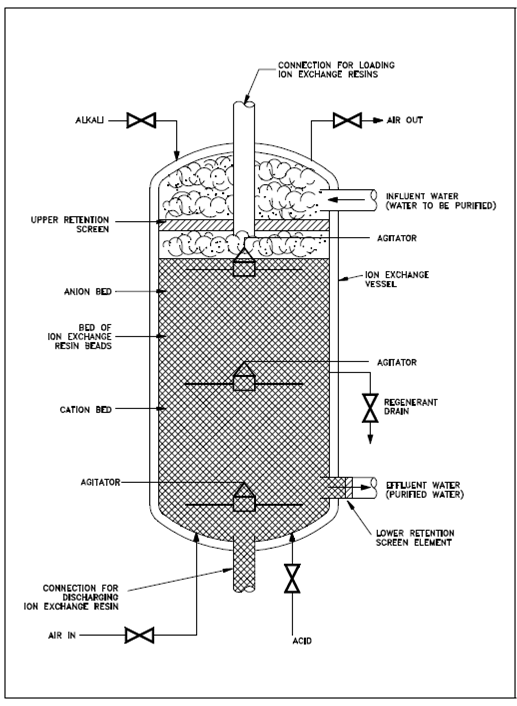
Figure: Schematic Diagram of a Typical Ion Exchanger
Because of the variant densities of anion and cation resins, a flow of solution (impure water) is from top to bottom. The lighter anion resin would gradually rise to the top through a procedure known as classification if the flow were reversed, resulting within a layer of anion resin on top of the cation resin, as displays in Figure. In the instance display, the layering output from regeneration and/or backwash. In systems not using a backwash, an anion and cation resin beads are uniformly varied. Several systems use a backwash process, if the resins are regenerated, to erase solids collected through filtration and to divide the resins for regeneration. That is remixed after reproduction.
For fixed amounts of cation and anion resins, the effectiveness for removal of impurities is greater in a mixed-bed resin than a layered arrangement. The major purpose is that for layered resins there might be huge pH gradients inside the column of resin. If, for instance, the hydroxyl form resin is on top, as solution passes by it anionic impurities are erased and replaced through OH- ions; therefore, the pH increases. This increase in pH might decrease the effectiveness within lower portions of the resin bed for erasing impurities. It might also cause a few impurities to precipitate since solubility changes along with pH. The resin column will filter a few undissolved materials, other than the effectiveness for filtration is commonly significantly less than which for removal through ion exchange. Therefore, the whole effectiveness is less than in a mixed-bed resin.