Chemical processes:
The chemical processes included in producing anion and cation resins are outlined in Figures, starting along with the created of cross-linked polystyrene. The polymer itself is a covalent compound. Through the chemical reactions denoted in Figure, hydrogen atoms covalently bonded to the original polymer at many sites are replaced through functional groups (called radicals) such as SO3H (sulfonic acid) and CH2N(CH3)3Cl (quaternary ammonium). Every such group is covalently bonded to the polymer, other than each also holds an atom which is bonded to the radical group by a predominantly ionic bond. In the two examples above, H in SO3H and Cl in CH2 N (CH3)3Cl are the ionically-bonded atoms. Many times these are written as SO3- H+ and CH2N (CH3)3 +Cl- to emphasize their ionic characters. Those ions (H+ and Cl-) are replaceable through other ions. That is, H+ will exchange along with other cations within a solution, and Cl- will exchange along with other anions.
In its last form, an ion exchange resin contains a large, but finite, number of sites occupied through an exchangeable ion. The all resin, except the exchangeable ion, is inert within the exchange procedure. Therefore, it is customary to use a notation like as R-Cl or H-R for ion exchange resins. R denotes the inert polymeric base structure and the category of the substituted radical which does not participate in exchange reactions. The word R is inexact since it is used to represent the inert portion of both cation and anion resins, that are slightly different. In addition, the structure represented through R holds several sites of exchange, while only one is displays through the notation, like as R-Cl. Despite these disadvantages, the word R is used for simplicity.
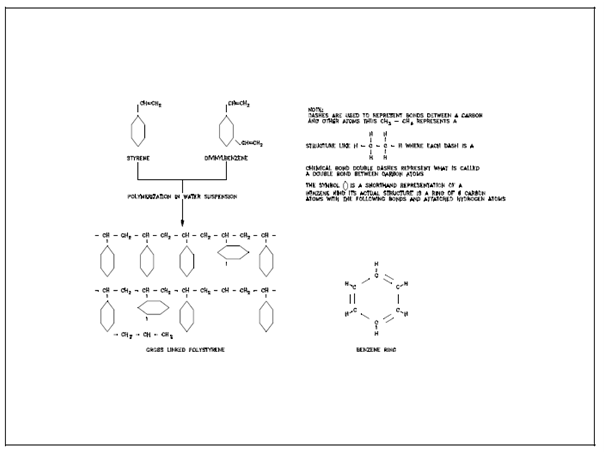
Figure: Polymerization of Cross-Linked Polystyrene Resins