Water Purity
The water used in a nuclear facility has to be of a purity level which is consistent along with the whole objectives of chemistry control within the facility.
There are a number of ways in that pure water is acquire, involving distillation systems and pretreatment systems same to those mentioned previously in this module. Regardless of the method employed, the needed purity must be getting.
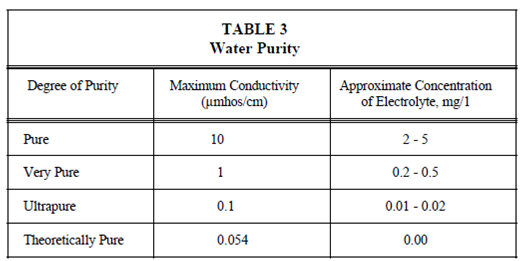
Water purity has been defined in several different ways, but one common accepted definition states which high purity water is water which has been distilled and/or de-ionized so in which it will have a exact resistance of 500,000 ohms (2.0 micromhos conductivity) or greater. That definition is satisfactory as a base to work from, other than for more critical needs, the breakdown displays in Table 3 has been optional to express degrees of purity.
Conductivity is a measure of the ease along with that electricity could be passed by a substance. The presence of ions greatly facilitates the passage of an electric current. The Pure water is just slightly ionized through the dissociation of water: H2O→ H++ OH-. At 25º C, the concentration of the hydroxyl ions and hydrogen is 10-7 moles/liter.
The equivalent conductance of hydrogen (H) is
350 (mhos- cm2/ equivalent)
and the equivalent conductance of OH is
192 (mhos- cm2/ equivalent)