Channel molecular biology
By sequencing and cloning the DNA which codes for a protein then it is possible to deduce its main amino acid sequence. This gives clues as to the secondary structure of the protein like the presence of α-helical or β-pleated sheet areas.
Hydropathicity plots, that represent how hydrophobic or hydrophilic specific amino acid stretches are, indicate which areas of the protein may be positioned in the membrane. Recognizing consensus series for glycosylation or phosphorylation permit specification of extracellular and intracellular areas of the molecule correspondingly, given that evidence for how a protein is orientated in the membrane.
Site-directed mutagenesis, in which the DNA coding for protein is modified in accurate ways by using genetic engineering methods and the mutated protein consequently expressed in several convenient ways, can be used to investigate the functions of specific amino acids in a channel.
The 3-dimensional structure of ion channels in both closed and open states can be deduced by X-ray crystallography from which the details of how the channel operates can be worked out. The main difficulty here is being able to crystallize the proteins.
There are several types of voltage-dependent ion channel, structures for a lot of of which have been deduced by using the methodologies outlined above. The sodium channels and numerous potassium channels, involving the delayed outward rectifier responsible for the down stroke of the action potential, share a common structure. The fundamental subunit has six highly hydrophobic segments (S1–S6), thought to be α-helices spanning the membrane as shown in fgure.
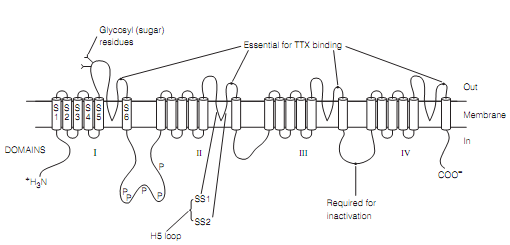
Figure: A diagram depicting the secondary structure of a Nav.The Segments S1–S6 are labeled only in domain I. P, consensus series for phosphorylation.
In the situationof potassium channels every subunit is an individual molecule and a functioning channel is a tetrameric homo-oligomer, which is, it consists of four similar subunits set around the central pore. At sodium channels the four subunits are linked by the cytoplasmic loops to form a single huge molecule; however its tertiary structure is thought to resemble a potassium channel.
The Segments 5 and 6 and the H5 loop among them contribute to the pore. This H5 loop is cosidered to line the channel pore as it contains amino acids which are crucial for conferring ion selectivity. The oxygen atoms are used to extracellular throat and is lined with which are crucial for ion permeation.
One of the most striking features of voltage-dependent ion channels is the S4 segment as shown in figure. It is highly conserved among channels, suggesting it has hardly modified during the evolution and therefore serves a critical function. However mostly hydrophobic, its N-terminal end has many positively charged lysine or arginine residues. The Site-directed mutagenesis displays that S4 is a part of the voltage sensor needed for activation of the channel.
The X-ray crystallography implies that in response to depolarization, the S4 segment acts like a paddle swinging via the membrane towards the extracellular side, as its positive charges are repelled by the voltage change. This displacement of the paddle pulls an S4–S5 linker area, opening the intracellular throat of the pore.
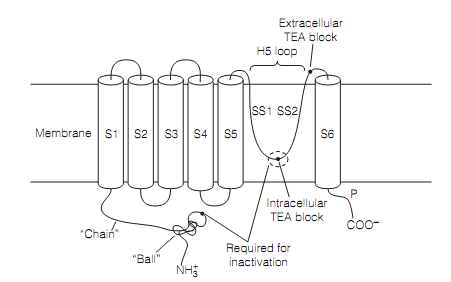
Figure: A diagram depicting the secondary structure of an outward rectifying potassium channel subunit. Four subunits bring together to form a functioning channel.
The three mechanisms to close the potassium channels have been proposed. One, N- inactivation happens by the so called ball and chain mechanism. This includes a cluster of amino acids (i.e., the “ball”) swinging up to block the internal mouth of the channel by interacting with an amino acid at the internal tip of the H5 loop. In Drosophila channels this peptide is the N-terminus of the standard (α) subunit. In mammals the ball is the N-terminus of an individual β-subunit.