Structural analysis:
The λmax for an absorption related to a specific electronic transition depends upon the extent of conjugation in the molecule and as well the kind of substituent that might be present. The predicted value for λmax can be measured by using tables that quantify these effects for a specific parent system, for example the table for conjugated dienes is displayed in figure.

Figure: Table for conjugated dienes.
The supposed λmax for the triene in figure is calculated as follows. The parent system is the conjugated diene (214 nm). There is one additional double bond in conjugation with the diene that means adding an additional 30 nm for double bond extension. There are as well substituents attached to the triene system - three methyl groups worth 5 nm each, and one methoxy group worth 6 nm. This provides a predicted λmax of 265 nm.
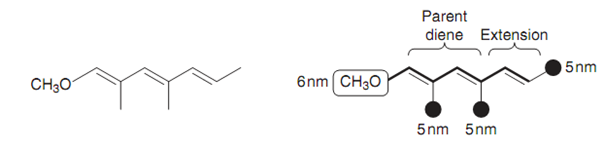
Figure: Theoretical lmax for a triene.
A comparison of the expected λmax versus the observed λmax can be employed as supporting proof for a proposed structure. It can also be helpful in choosing among two or more likely structures for a test compound. Separate tables are obtainable for α, β-unsaturated aldehydes and ketones, α, β-unsaturated acids and esters, and benzene derivatives.