Plaque assay
The plaque assay quantifies the number of infectious units (in this instance, plaque forming units) in a given suspension of virus. Plaques are localized discrete foci of infection denoted by zones of cell lysis or cytopathic effect (CPE) within a monolayer of otherwise healthy, cultured cells. Because of the great dilution at which viruses are assayed, each plaque in theory originates from a single infectious virion having infected a single cell, thus allowing a very precise calculation of the virus titer. The most common plaque assay is the monolayer assay. Here, a small volume of virus diluent (0.1 ml) is added to a previously seeded subconfluent cell culture monolayer. Following adsorption of virus to the cells, a semi-solid overlay (usually medium containing agar or agarose) is added to prevent the formation of secondary plaques, by restricting the range of movement of virions released from the infected cells, so that only adjacent cells within the monolayer are infected. Over a number of days, uninfected cells divide until the monolayer is near or at confluence, while virus replication causes small, discrete areas of infection and CPE that slowly expand as new virions infect neighboring cells, forming the plaque. Following incubation, the cell monolayers are usually fixed (a process that kills the cells but stabilizes their morphology and position) using alcohol- or formaldehyde- based solvents and stained so that infected and uninfected cells can be distinguished. Common stains include crystal violet (which stains infected cells, giving dark plaques) and neutral red (which leaches out of infected cells rapidly, giving pale plaques against a darker pink background of uninfected cells).
The plaques are counted within each plate and the counts for plates at one of the virus dilutions are used to calculate the original virus titer. The choice of dilution depends on both the certainty of the plaque count (i.e. high enough that plaques are discrete/not overlapping) and also the need for statistical reliability (i.e. enough plaques to be considered reliable). For these reasons choosing a dilution where each replicate of the assay holds between 20 and 100 plaques per monolayer is ideal, although the actual number that can be easily counted is often dependent on the size of the plaque and the size of the vessel used for the assay. Typical plaques are shown in Figure, and the assay procedure is summarized in another figure. The
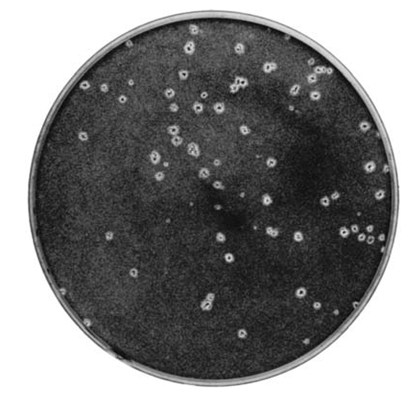
Figure: Herpes virus plaques on a tissue culture monolayer.
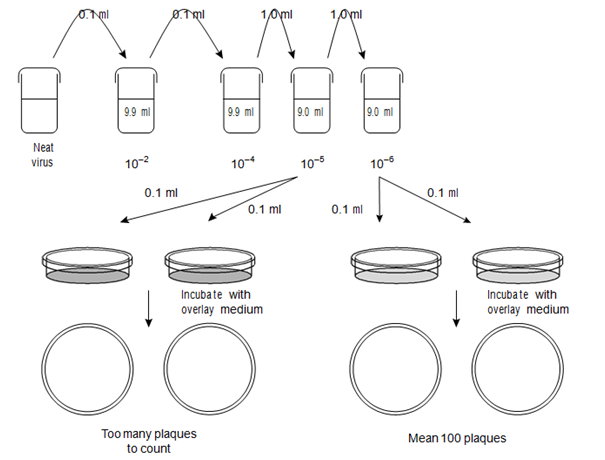
Figure: Diagrammatic representation of virus plaque assay (see text for calculation).
infectivity titer is expressed as the number of plaque forming units per ml (pfu ml-1) and is obtained in the following way:
pfu ml-1 = (mean number of counted plaques)/(dilution * volume (ml) tested).
For example, if there is a mean number of 100 plaques from monolayers infected with 0.1 ml of virus that has been diluted to a level of 10-6 then the calculation is:
pfu ml-1 = (100)/(10-6 * 0.1) = 100/10-7 = 100 * 107 = 1 * 109 pfu ml-1