The Carnot Vapor Cycle
The Carnot vapor cycle is employed as an ideal power cycle for steam power plants. Let consider an ideal Carnot cycle as shown in the figure shown below. Here the functioning fluid is water, in the cyclic procedure 1-2, water is heated reversibly and isothermally in boiler, in the procedure 2-3 it is expanded isentropically in a turbine, in the procedure 3-4, then expanded water is condensed isothermally and reversibly in a condenser and lastly in the procedure 4-1, the fluid is compressed isentropically by a compressor to the original state.
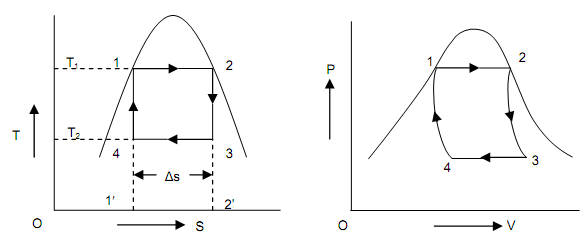
Figure: Carnot Cycle on P-V and T-S Diagram
The four Carnot cycle procedures are as follows:
• Isothermal heat addition from procedure 1-2,
• Isentropic expansion of steam in an expander from procedure 2-3,
• Isothermal heat rejection in the condenser from procedure 3-4, and
• Isentropic compression of a mixture of vapor and liquid from procedure 4-1.
By taking 1 kg of water as working fluid for a vapor power cyclic procedure, Heat added = Q1, that is shown on T-S diagram as region (1-2-2′-1′-1) = T1 ΔS,
ΔS = change in entropy.
Heat added = Q2, region (3-2′-1′-4-3) on T-S diagram = T2 ΔS
Net Work done = W = Q1 – Q2 = T1 ΔS – T2 ΔS = (T1 – T2) ΔS
Thermal efficiency of the cycle = Work done/ Heat added
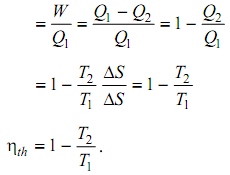
Limitations of Carnot Cycle:
Carnot vapor power cycle is an ideal cycle, efficiency of which is self-governing of the working substance,
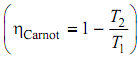
Though it is tremendously hard to operate in practice since of the reasons as follows:
(a) It is very difficult to compress a wet vapor isentroprically to the saturated state as needed by the procedure (4-1).
(b) It is very difficult to control the quality of the condensate coming out of the condenser and hence the state ‘4’ is exactly acquired.
(c) The effectiveness of the Carnot cycle is properly attached by the temperature T1 at which heat is transferred to the working fluid. As the temperature of steam is merely 374oC, hence, when the cycle is to be function in the wet area, the maximum possible temperature is harshly limited.
(d) Isentropic compression of a vapor needs more work due to its high specific volume therefore decreasing the work ratio.
(e) Isothermal heat addition subsequent to the saturated vapor line is very hard to attain as it includes heat addition at the same time of expansion steam.
Due to all above reasons, it is not possible to attain the high efficiency as derived by the Carnot vapor cycle.