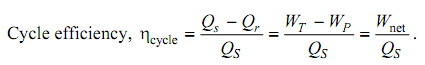Binary Vapor Cycle
In the vapor power cycles most generally used functioning fluid is water. Though at high temperatures to acquire high efficiency of vapor power cycle, some other working fluids are employed. At high temperatures few working fluids are employed, that are sodium, mercury, potassium and sodium-potassium mixtures. Amongst these, only mercury has been employed in practice.
For best performance, the working fluid must have the characteristics shown below:
• High Critical temperature and safe maximum pressure,
• Low triple point temperature,
• Condenser pressure that is not too low,
• High enthalpy of vaporization,
• Good heat transfer features, and
• Inert, easy accessibility at low cost.
To raise the efficiency of Carnot cycle, with a rise in initial temperature or with decrease in exit temperature of the fluid. At normal pressure of 12 bar, the saturation temperature for water and mercury are 187oC, – 560oC, correspondingly. The maximum temperature attained in power plants is around 550 – 600oC. Hence mercury is a better functioning fluid in the high temperature range, since its vaporization pressure is associatively low. Mercury vapor at high temperature with low pressure that avoids the complexities connected with high pressure.
To acquire the high thermal efficiency of the power plant, by employing two functioning fluids like water and mercury, the binary vapor cycle has been build up. The power cycle, that is a combination of two cycles, one in the high temperature area and the other in the low temperature area, termed as the binary vapor cycle. In this cycle, the condenser of high temperature cycle termed as the tapping cycle serves as the boiler of the low temperature cycle, known as the bottom cycle. Mercury water binary vapor cycle with T-S diagram is as shown in figure below.
Cycle Efficiency Calculation:
For evaluating the efficiency of binary vapor power cycle, we should draw the temperature (T), entropy (S) diagram. In this diagram it contains mercury cycle and steam cycle. The mercury quits the condenser as saturated liquid and steam leaves as saturated vapor. The mercury cycle 1-2- 2′-3-4-1 is termed as topping cycle and steam cycle 5-6-6′-7-8-5 as the bottoming cycle. The mercury departs the condenser as saturated liquid and steam leaves as the saturated vapor. The condensed mercury liquid is pumped reverse to its boiler with the aid of mercury pump. Evaporated steam is super-heated in the boiler, after being sent to economizer. It is then extended isentropically in the steam turbine to a point 6; lastly the steam is condensed in the steam condenser up to point 7 and pumped to the steam boiler.
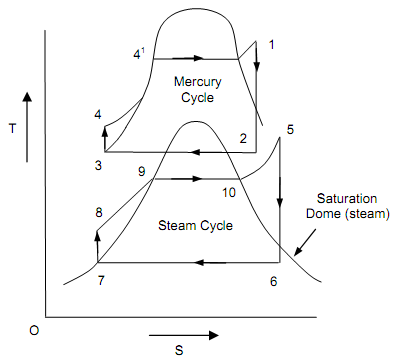
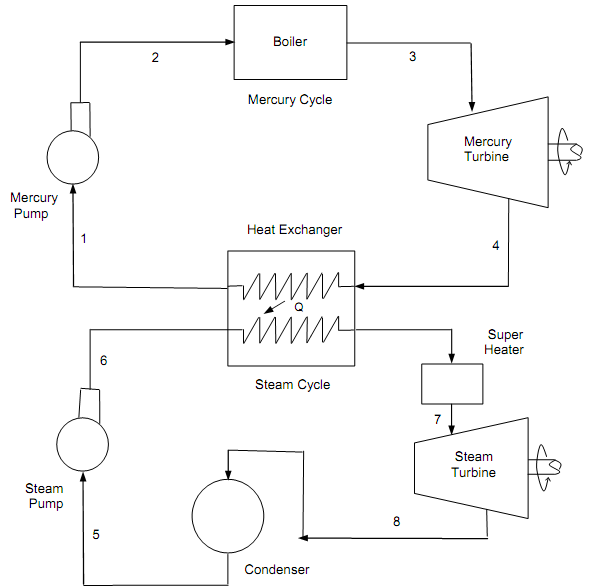
Figure: Mercury Water Binary Vapor Cycle
Assume 1 kg of steam be evaporated in the mercury condenser and it needs mhg of mercury vapor. The energy balance for mercury condenser can be illustrated as:
mhg = (h2 – h3) = 1 x (hf - he)
Or, mhg = (hk - hf) / (h2 – h3)
By ignoring pump work,
mhg x x2 hfg2 = 1 x (hf - he) = 1 x hfg
Network completed per kg of steam,
Wnet = (Mercury turbine work) – (Mercury pump work) + (Steam turbine work)
– (Steam pump work)
= mhg (h1 – h2) – mhg (h4 – h3) + (h5 – h6) + (h8 – h7)
When the pump work is neglected,
Wnet = mhg (h1 – h2) + (h5 – h6)
The heat supplied per kg of functioning fluid,
Qs = mhg (h1 –h4) + (h5 – h10) + (h9 – h8)
The heat discarded per kg of working fluid (steam),
Qr = (h6 – h7)