Standard addition method:
Sometimes, it might so happen that all the constituents in the sample might not be known; so a standard solution along with the similar chemical composition cannot be prepared. In such a case, a method known as standard addition method is used that eliminates any error arising from molar absorptivities (ε) being different in the standard solution from which in the sample solution. Within this method known amounts of the standard is added to identical aliquots of the sample and the absorbance is measured. The first reading is the absorbance of sample alone and the second reading is absorbance of sample holding analyte plus, a known amount of analyte and many more. The readings so acquired are then plotted to acquire the calibration curve. If the Beer's law is obeyed, that is a straight line is acquired; the unknown concentration of the solution could then be acquired through the extrapolation of the calibration curve as displays in Figure (b).
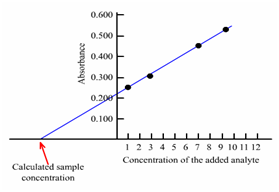
Figure: Calibration curve: b) Standard addition method