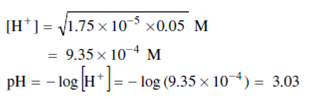Titration of a Weak Acid with a Strong Base:
So far titration curve shown in Figure (a) describe the progress of the titration of strong acid and strong base. We now assume the titration of 25 cm3 of 0.05 M CH3COOH solution with 0.05 M NaOH solution to explain how the pH of the titration is changed at different stages of the titration. For this purpose we will be using many expressions used in theory of neutralization titrations which you may have studied in first course on 'Basics of Analytical Chemistry or in your undergraduate physical chemistry courses.
Starting point of the titration curve: The starting point of the titration curve of 0.05 M CH3COOH solution is considerably lower than that of 0.05 M HCl solution. This is because acetic acid is dissociated almost by 100 times less than hydrochloric acid. (the degree of electrolytic dissociation of 0.05 M CH3COOH solution α ≈ 1 per cent; for 0.05 M HCl solution, α ≈ 90 per cent). Hence [H+] in 0.05 M CH3COOH solution is also 100 times less than within 0.05 M HCI solution. Or pH will be 3 and not unity.
A more precise pH value of the starting point of the titration curve is found by the following method. Write the expression for acid dissociation constant for acetic acid:
Ka = [H+][A-]/[HA]
At equilibrium, [H+] = [A-]. As the degree of ionization of acetic acid is small, therefore at equilibrium, the concentration of unionized acid is approximately equal to total concentration of acid (cacid), i.e. cacid = [HA] + [A-] ≈ [HA]. Thus,
Ka = [H+]2/[HA] = [H+]2/cacid
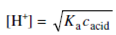
here cacid is the concentration of acidic acid.
For acetic acid Ka = 1.75 × 10 -5