Indicator glass electrode:
As described in Figure (a), these electrodes have thin glass membrane fused to the end of a glass or plastic body. The primary body of the electrode holds an internal reference electrode classically Ag/AgCl and is filled along with a solution which is commonly the aqueous HCl of concentration around 1.0 mol dm -3. The selectivity coefficient of glass-membrane is such that excellent dissemination against common cationic species is achieved.
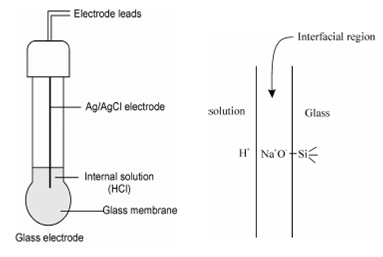
(a) (b)
Figure: Glass electrode: (a) Typical glass electrode consisting of both an indicator glass electrode and a silver/silver chloride reference; (b) illustration showing an ion exchange
The pH electrode responds to hydrogen ions as a result of the thin ion-exchange sites on the surface of a hydrated glass membrane. An electrode consists of a thin layer of glass, typically about 50 µ m thick. Charge is transported across the membrane by sodium or lithium ions within the glass. The membranes are primarily made from Lithia (Lithium oxide) or sodium oxide, and SiO2, and some amount of Al2O3 and B2O3 or multi-component glasses whose sensitivity pattern depends on the composition of the glass. A surface layer of the glass consist of silicate group associated with sodium ion (- Si O- Na+) as shown in Figure (b). When this electrode is dipped in water and the sodium ions exchanged with the protons in water.
- Si O- Na+ + H+ → - SiO - H+ +Na+