Evaluate the pH during the titration:
i) The pH of a solution of NaCN can be calculated as
CN - + H2O ↔ HCN + OH-
Kb = [OH-][HCN]/[CN-] = Kw/Ka =1.00×10-14/6.2×10-10 =1.61×10-5
Sinceanequ ivalentamountof [OH-] and [HCN] areformed
[OH- ] = [HCN]
[CN-] = c NaCN - [OH-] » c NaCN = 0.05M
Substitution into the above dissociation-constant expression provides, after rearrangement,
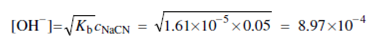
pH = 14.00 - (- log 8.97×10 -4 ) 10.95
ii) 10.00 cm3 of Reagent
Addition of acid generates a buffer along with a composition given through
cNaCN =50.00×0.05-10.00×0.100/60.00 = 1.50/60.00 M
cHCN = 10.00×0.10/60.00 = 1.000/ 60.00 M
Those values are then substituted within the expression for the acid dissociation constant of HCN to give [H+] directly:
[H+]=Ka×cacid/csalt
[H+] = 6.2×10-10×(1.000/60.00)/1.50/60.00 = 4.13×10-10
pH = -log4(4.13×10-10)=9.38
iii) 25.00 cm3 of Reagent
This volume corresponds to the equivalence point, where the principal solution species is the weak acid HCN. Therefore,
cHCN =25.00×0.10/75.00 =0.033M
Applying following Equation gives
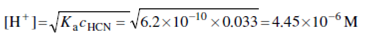
pH = -log(4.45×10-6)=5.34
iv) 26.00 cm3 of Reagent
The excess of strong acid now present represses the dissociation of the HCN to the point where its contribution to the pH is negligible. Thus,
[H+] = cHCI = (26.00×0.10-50.00×0.05)/76.00 =1.32×10-3M
pH= -log(1.32×10-3)=2.88