Equivalence Point:
We can also determine the pH at the equivalence point. At the moment when titration is finished, equivalent quantities of the CH3COOH and NaOH solution will be present. Therefore, the titrating flask will have a salt solution formed by a weak acid and a strong base. For the solution of such salt (see marginal remark):
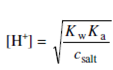
The salt concentration in comparison with 0.05 M will halve;
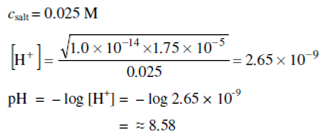
Intermediate Points: After determining the starting and end points of the titration, we will now consider pH calculations for the intermediate points. These points of the curve correspond to the simultaneous presence in the solution of an un-titrated weak acid and a salt which is formed as a result of its partial neutralization. Hence, for calculation, we may use the formulas for finding the values of [H+] and pH in solutions of a weak acid in the presence of its salt of strong base (see marginal remark):
[H+] = Ka (cacid/csalt)
Let us calculate the first intermediate point which corresponds to 5 cm3 of 0.05 M NaOH solution poured in. We first determine the quantity (in cm3 of 0.05 M solution) of the residual acid. It will be 25 - 5 = 20 cm3, because 5 cm3 of 0.05 M NaOH solution have titrated CH3COOH. As result, cacid in the titration flask is not 0.05M but
cacid = (0.05 × 20)/30 = 0.033 M
Let us now determine the concentration of the salt formed at this moment of titration. As we have seen previously, 5 cm3 of 0.05 M CH3COONa solution were build, but this quantity of the salt is also in the total volume of 30 cm3. Thus,
csalt = (0.05 × 5) /30 = 0.008 M
For acetic acid Ka is 1.75 × 10-5
Substituting all these data in the formula for finding the pH of the solution, we obtain the following for the first intermediate point:
[H+ ] = ((1.75 × 10-5 × 0.033)/ 0.008) M
pH = - log [H +] = - log 7.22×10-5
= 4.14