Curve of titration of a weak acid:
Thus, the curve of titration of a weak acid with a strong alkali has the following signs:
1. The starting point of titration is in a medium which is less acidic than when a strong acid is being titrated,
2. the equivalence point is in a weakly alkaline medium,
3. the middle part of the titration curve is more slanting than that of the titration curve of a strong acid;
4. the titration jump is not great ranging to pH 8 to pH 10, and accordingly, the vertical part of the curve is considerably smaller than that when a strong acid is being titrated.
Buffering action of the CH3COO- ion: One will see without any difficulty that this curve is much smoother than the curve of titration of a strong acid with a strong alkali, and it does not have a sharp inflection.
While HCl solution was titrated with alkali, 22 cm3 of the titrant had to be added in order to change pH by unit, while in this case, approximately 5 cm3 were enough (Figure).
This difference is because of the fact that when titrating a strong acid with a strong alkali, the concentration of H+ ions decreases only as a result of their combination with OH- ions.
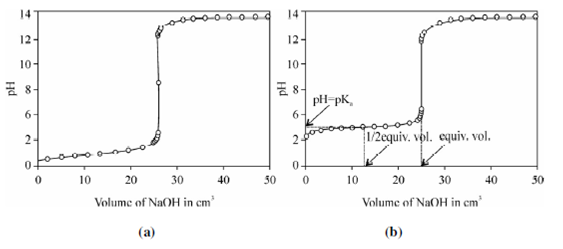
Figure: pH titration curves: (a) For 0.50 HCl with 0.50 NaOH; (b) 0.50 Acetic acid with 0.50 NaOH.