Calculate the pH during the titration:
Calculate the pH during the titration of 50.00 cm3 M NaOH with 0.10 M HCl after the addition of the following volume of acid: (i) 24.50 cm3, (ii) 25.00 cm3 and (iii) 25.50 cm3.
i) At 24.50 cm3 added, [H+] is very small and cannot be computed from stoichiometric considerations but can be obtained from [OH- ]
[OH- ] = cbase = (Original no. mmol NaoH - No. mmol HCl added)/Total volume of solution
=50.00×0.05-24.50×0.10/50.00+24.50 = 6.71× 10-4 M
[H+] = Kw /(6.71×10-4)=1.00×10-14 /(6.71×10-4)
= 1.49×10-11 M
pH = - log(1.49×10-11 M) =10.83
ii) That is the equivalence point where [H+]= [OH-]
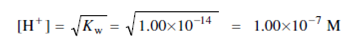
pH = - log (1.00 ×10 -7 ) = 7.00
iii) At 25.50 cm3 added,
[H +] = cHCl = ((25.50 × 0.10 - 50.00 × 0.05)/75.50)
= 6.62 × 10-4 M
pH = - log (6.62 ×10 -4 ) = 3.18