Iron oxidation
Bacteria such as Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans and the Archaea Ferroplasma are able to grow using reduced iron as an electron source gaining carbon from the atmosphere. These iron oxidizers grow at low pH and are frequently found in high numbers in acid mine run off. Here, oxygen is low and iron high. The energy from the oxidation of iron (II) to iron (III) in Figure 2 is enough to drive ATP synthesis when coupled to some of the components of the electron transport system. The iron oxidation system is responsible for the deep red color found in many mine-polluted rivers and streams. The Phototrophic bacteria can also perform a similar reaction at higher pH, coupling iron oxidation to sulfide oxidation or even denitrification.
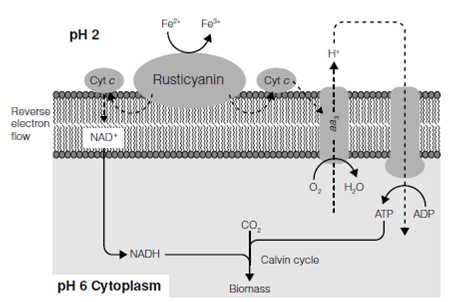
Figure . Microbial iron oxidation. Cyt c, cytochrome c; aa3, cytochrome aa3 oxidase.