Weak Acid along with a Strong Base, example for acetic acid within NaOH:
Initially the conductance is low because of the feeble ionization of acetic acid. At the addition of base, there is reducing within conductance not only because of the replacement of H+ through Na+ but also suppresses the dissociation of acetic acid due to general ion acetate. But extremely soon, the conductance increases on adding NaOH as NaOH neutralizes the un-dissociated CH3COOH to CH3COONa that is the strong electrolyte. This rise in conductance continues raise up to the equivalence point. The graph near the equivalence point is curved because the hydrolysis of salt CH3COONa. Beyond the equivalence point, conductance increases more rapidly along with the addition of NaOH because of the highly conducting OH- ions display in the below figure.
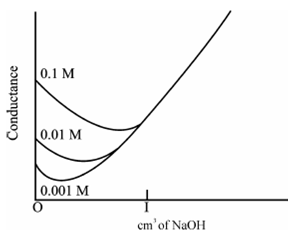
Figure: Conductometric titration of a weak acid (acetic acid) vs. a strong base (NaOH)