Displacement (or Replacement) Titrations:
While a salt of a weak acid is titrated along with a strong acid, a anion of the weak acid is exchanged through that of the strong acid and weak acid itself is liberated within the undissociated form. As same, further of a strong base to the salt of a weak base, a cation of the weak base is replaced through that of the stronger one and the weak base itself is produced in the undissociated form. If for instance, M-HCl is added to 0.1 M solution of sodium acetate, the curve displays in Figure is acquire, the acetate ion is replaced through the chloride ion after the endpoint. An initial rise in conductivity is because of the fact in which the conductivity of the chloride ion is slightly greater than which of acetate ion. Until the replacement is nearly finished, the solution contains sufficient sodium acetate to suppress the ionization of the liberated acetic acid, so resulting a negligible increase in the conductivity of the solution. Therefore, near the equal point, the acetic acid is satisfactorily ionized to affect the conductivity and a rounded portion of the curve is obtained. Beyond the equivalence point, while excess of HCl is present (ionization of acetic acid is extremely much suppressed) thus, the conductivity arises rapidly. Care must be taken that to titrate a 0.1 M-salt of a weak acid, a dissociation constant should not be more than 5×10-4, for a 0.01 M -salt solution, Ka < 5 ×10-5 and for a 0.001 M-salt solution, Ka < 5 ×10-6, i.e., the ionization constant of the displace acid or base divided by the real concentration of the salt must not exceed above 5 ×10-3. Also involves the titration of 0.01 M- ammonium chloride solution versus 0.1 M- sodium hydroxide solution. The reduction in conductivity during the displacement is caused through the displacement of ammonium ion of grater conductivity through sodium ion of smaller conductivity.
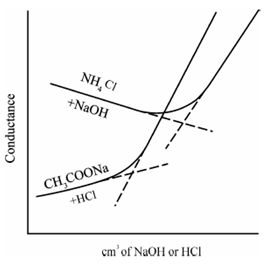
Figure: Conductometric titration of a salt of weak acid (sodium acetate) vs. strong acid (HCl); salt of a weak base (NH4Cl) vs. a strong base (NaOH)