Potential changes in during an electrolysis at constant applied potential:
Let Consider electrolysis of Cu (II) ion in acid medium. Initially the applied potential to the cell is about - 2.5 V, that leads to a current of about l.5 A as display in Figure. The electrolytic deposition of copper is then finished at this applied potential. As the reaction proceeds, IR decreases continually. This decrease is because of concentration polarisation at the cathode that limits the rate at that copper ions are brought to the electrode surface and therefore limits the current. The decrease in IR must be compensated through an increase within the cathode potential because the cell potential is constant. The increase within cathode potential is slowed down at point B through the reduction of hydrogen ions. Because the solution contains a large excess of acid, a current is no longer limited through concentration polarisation, or co-deposition of copper and hydrogen goes on at the same time until all the copper ions are deposited. Below these conditions, the cathode is said to be depolarised through hydrogen ions.
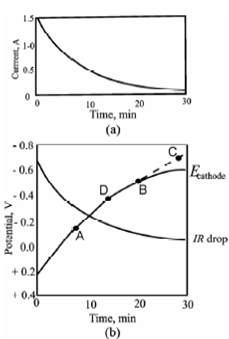
Figure: (a) current, (b) IR drop, and cathode-potential change during the electrolytic deposition of copper at a constant applied cell potential.