Constant Potential Electrolysis:
Through controlled potential electrolysis, it is probable to separate two elements whose deposition potentials differ suitably (through a few tenths of a volt). The potential of the cathode is controlled so in which it never becomes suitably negative to permit the deposition of the further element. As can be seen from the Figure, the potential of the cathode becomes negative (because of concentration polarisation) and which co-deposition of the other species begins before the analyte is fully deposited. A huge negative drift in the cathode potential can be prevented through using a three electrode system as display in Figure.
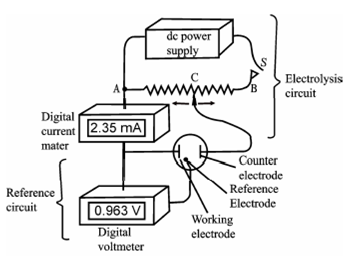
Figure: Apparatus for controlled-potential electrolysis. Contact C is adjusted as necessary to maintain the working electrode (cathode in this example) at a constant potential. The current in the reference-electrode circuit is essentially zero at all times