Stepwise Line Fluorescence:
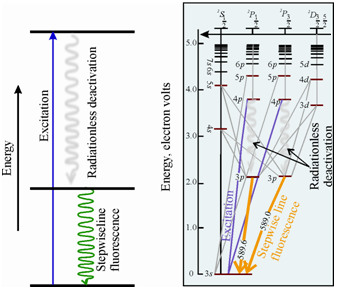
In this kind of fluorescence an atom initially excited to a higher energy state through absorption of radiation, undergoes deactivation through a radiationless procedures to a lower excited state, from that it emits radiation to return to the ground state. It is also a category of Stokes fluorescence. The schematic energy level diagram displaying the origin of stepwise such as fluorescence is given in Figure(a).
(a) (b)
Figure: Schematic representation of (a) Energy transitions involved in stepwise line fluorescence and (b) Grotrian diagram of sodium atom showing the origin of stepwise fluorescence line
The fluorescence emission through sodium atom is an instance of stepwise line fluorescence, as display in Figure (b). In sodium atom a 3s electron is excited to 4p level by a 330.2 nm radiation. This electron then relaxes down to an intermediate level (3p) within a radiationless procedure. It is the fluorescence emission at 589.0 nm. The additional relaxation from this level is nonradiative in nature.