Resonance Fluorescence:
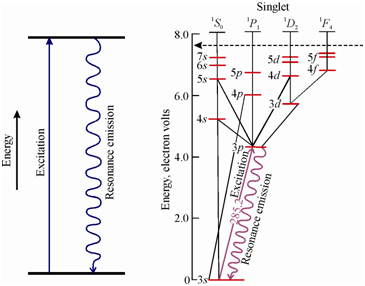
Resonance fluorescence occurs while the excited states emit a spectral line having the similar wavelength as in which used for excitation. Figure (a) provides the origin of resonance fluorescence line within terms of a schematic energy level diagram.
(a) (b)
Figure: Schematic representation of (a) Energy transitions involved in direct line fluorescence spectral line and (b) Grotrian diagram of thallium atom showing the origin of direct line fluorescence
Thallium atom is an instance of an atom showing direct line fluorescence. Let consider the energy level diagram of thallium atom displays in Figure (b). You could observe in which while excited through a radiation having a wavelength of 377.6 nm, the thallium atom returns to the ground state within two steps producing a fluorescence emission line at 535.0 nm followed through radiationless deactivation.