Vertical trends
The IE usually decreases down each group of elements. Diagram 2 depicts this for hydrogen and the elements of group 1, all of which comprise the (ns)1 outer electron configuration. The main affect here is the increasing value of principal quantum number n. The fall in IE is, though, much less steep than the simple hydrogenic prediction (1/n2;). There is a considerable increase of nuclear charge among each element, and even though extra inner shells are taken, they do not give perfect shielding. So, contrary to what is sometimes stated effective nuclear charge increases down the group. In the resultant balance between increasing n and increasing Zeff (see Equation 1) the former usually dominates, like in group 1. There is, though, nothing inevitable about this and there are occasions in later groups where Zeff increases adequately to cause an increase of IE between an element and the one below it. Diagram 2 also displays the group 11 elements Ag, Cu, and Au, where an ns electron is also being ionized. The increment of IE along period 4 between K (Z=19) and Cu (Z=29) is caused due to the extra nuclear charge of 10 protons, partly protected by the 10 added 3d electrons. A similar increase takes place between Rb and Ag in period 5. In period 6, though, the 4f shell intervenes giving 14 additional elements and leading to a total increase of Z of 24 between Cs and Au. There is a much more considerable increase of IE so, and Au has a higher IE than Ag. (Relativistic effects also contribute) likewise irregular trends in IE may have some affect on the chemistry of p-block elements.
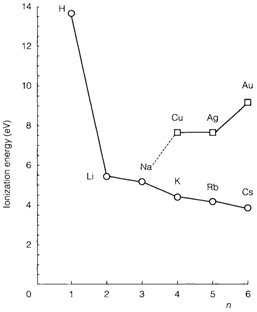
Orbital radii also rely on n2 and usually increase down each group. Since the radius depends on Zeff and not on irregular variations in this quantity have less affect than they do on IEs. There is other interesting characteristic of vertical trends, arising also from the way where the periodic table is filled.
For orbitals of a given l there is a more important change, both in IE and size, between the first and second periods involved than in consequent cases. Figure 2 demonstrates this for s orbitals, in which the IE decreases much more from hydrogen (1s) to lithium (2s) than among the lower elements. Such type of a distinction is reflected in the chemical properties
of group 1 elements, the hydrogen being nonmetallic and other element metals. Like, even though less dramatic, variations are found with 2p and 3d. So, period 2 p-block elements are in many ways distinct from those lower in the p block and 3d series elements different from those of the 4d and 5d series.