Energies and Sizes
The first ionization energy (IE) of an atom (M) is the energy needed to form the positive ion M+:
The IE value reflects the orbital's energy from which the electron is removed, and thus depends on the principal quantum number (n) and effective nuclear charge (Zeff;):
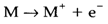
The average radius of an orbital depends on similar factors:
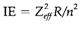
Smaller orbitals usually have more tightly bound electrons with higher ionization energies.
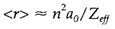
It is sometimes helpful to suppose that the distance among two neighboring atoms in a solid or molecule can be expressed as the sum of atomic or ionic radii. The Metallic, covalent or ionic radii can be described according to the type of bonding among atoms and vander Waals' radii for atoms in contact but not bonded. Such types of empirically derived radii are all dissimilar and are not easily related to any simple predictions based on isolated atoms. Though, they are, qualitatively associated to orbital radii and all follow the general trends