Elongation:
At the beginning of the first round of elongation, the initiation codon (AUG) is positioned in the P site with fMet-tRNAfMet bound to it through codon-anticodon base pairing. In The next codon the mRNA is positioned in the A site. The Elongation of the polypeptide chain occurs in three steps known as the elongation cycle which are namely aminoacyl-tRNA binding, peptide bond establishment and trans- location:
- Aminoacyl-tRNA binding: in this 1st step, the corresponding aminoacyl-tRNA for the second codon binds to the A site via codon-anticodon interaction. Binding of the aminoacyl-tRNA requires elongation factor EF-Tu and GTP that bind as an aminoacyl-tRNA/EF-Tu/GTP complex. Subsequent binding, the GTP is hydrolyzed and the EF-Tu is released, now bound to GDP. Before the EF-Tu molecule can catalyze the binding of another charged tRNA to the ribosome, it must be regenerated through a process including another elongation factor, EF-Ts. This regeneration is known as the EF-Tu-EF-Ts replace cycle. First, EF-Ts bind to EF-Tu and displace the GDP. Then GTP binds to the EF-Tu and displaces EF-Ts. An EF-Tu-GTP is now prepared to take part in another round of elongation.
- Peptide bond formation: the 2nd step peptide bond formation is catalyzed through peptidyl transferase. The carboxyl end of the amino acid bound to the tRNA in this reaction and in the P site is uncoupled from the tRNA and becomes joined through a peptide bond to the amino set of the amino acid connect to the tRNA in the A site. A protein with peptidyl transferase activity has never been isolated. The reason is now obvious; in E. coli at least, the peptidyl transferase activity is related with part of the 23S rRNA in the huge ribosomal subunit. Alternatively, peptidyl transferase is a ribozyme, a catalytic activity which resides in an RNA molecule.
- Translocation: in the 3rd step, a complex of elongation factor EF-G (also called translocase) and GTP example for EF-G/GTP binds to the ribosome. There are three concerted movements now occur collectively known as translocation; the deacylated tRNA moves from the P site to the E site, the dipeptidyl-tRNA in the A site shifts to the P site and the ribosome moves along the mRNA (5' to 3') through three nucleotides to place the next codon in the A site. In during the translocation events the GTP is hydrolyzed to GDP and inorganic phosphate and EF-G is released ready to bind more GTP for another round of elongation.
After translocation, the A site is blank and ready to receive the next aminoacyltRNA. The E site and the A site cannot be occupied concurrently. Therefore the deacylated tRNA is released from the E site before the next aminoacyl-tRNA binds to the A site to begin a new round of elongation.
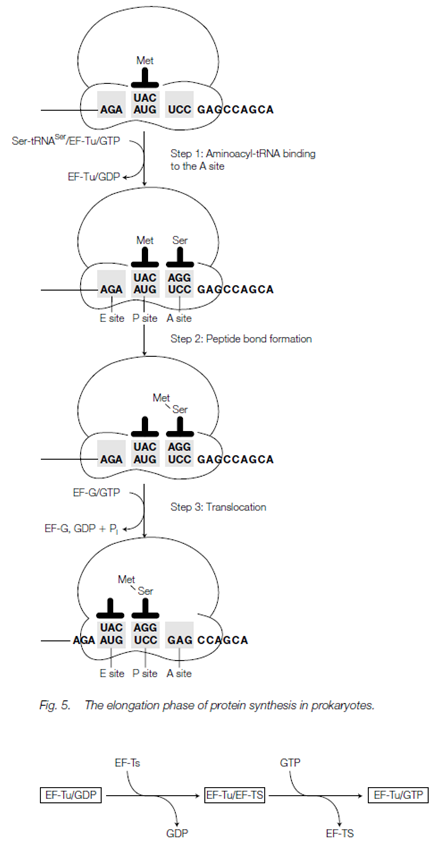
Figure: The EF-Tu-EF-Ts exchange cycle.
The Elongation continues adding one amino acid to the C-terminal end of the developing polypeptide for every codon which is read with the peptidyl-tRNA moving back and forth from the P site to the A site as it grows.
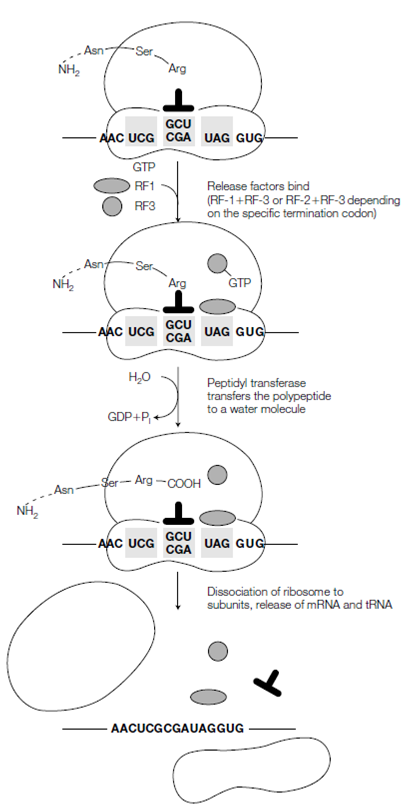
Figure: Termination of protein synthesis in prokaryotic cells.