Thermometric titraions possess:
Thermometric titraions possess extraordinary potentiality for determining heat of reaction in a dilute solution rapidly and conveniently so in which ?H values could be set virtually equal to the ideal thermodynamic parameters corresponding to infinite dilution.
Non aqueous systems are well suited for thermometric titrations though heat of mixing of solvent and dilution pose a problem. The lower specific heat of several organic solvents introduces a favorable sensitivity factor. Under strictly anhydrous conditions, even diphenylamine, urea, acetamide and acetanilide are readily titrated along with perchloric acid within glacial acetic acid. Lewis bases like as dioxane, morpholine, pyridine and tetrahydrofuran might be titrated with a Lewis acid such as SnCl4 in CCl4, benzene and nitrobenzene. TT is very meaningful for titrating acetic anhydride in acetic acid Sulfuric acid acetylating bath water in conc. acids through dilution along with fuming acids and free anhydrides in fuming acids.
Analysis of mixtures is probable while the two species have different equilibrium constants and heats of reaction with the titrant. For instance, in the titration of mixture of calcium and magnesium along with EDTA. Calcium (Kf = 1011) reacts first and exothermally (?H = -5.7 kcal/mole) and then magnesium (Kf = 109.1) reacts endothermally (?H = 5.5 kcal/mole) as described in Figure.
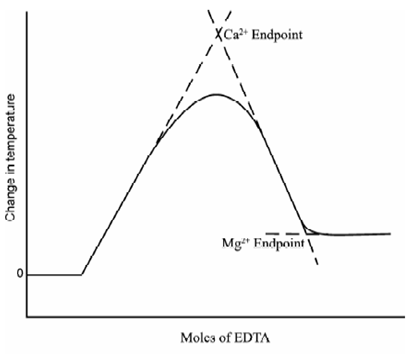
Figure: Typical curve for thermometric titration of a mixture of Ca2+ and Mg2+ with EDTA
Precipitation and ion-recombination reactions through TT also yield good results. For instance, halide reacts with silver or mercury (II), and cations such as Mn(II) with EDTA and oxalate. Titration of silver with halide can be carried out at elevated temperatures in molten state.