Principle:
Same to the case of thermogravimetry, primary principle of thermometric titrations is based on the change within temperature along with the addition of titrant and determine the end point from a plot of temperature vs. volume of titrant. The titrant is added to an isothermal titrate in an adiabatic titration calorimeter. In most examples there occurs a change in enthalpy concomitantly, yielding a corresponding heat of reaction. As a result these are also called enthalpy titrations and the titration curves are known as enthalpograms. It could be exothermic or endothermic. In thermometric titration, change in temperature occurs only while titration is in progress and sample reactant is present. Therefore, start and end point of a titration are readily observed and the number of moles titrated is calculated as in regular titration. Through determining the heat capacity of the system under study, heat of reaction could be readily determined. Further, equilibrium constant could be evaluated under suitable experimental conditions.
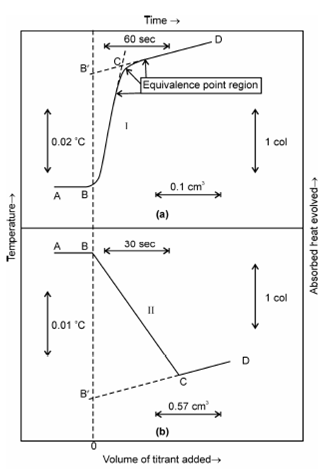
Figure: Tupical Enthalpogram for (a) exothermic reaction and (b) endothermic reaction, showing start of titration, progress of titration, end point and excess reagent line