Determine equilibrium constant of complex formation:
Determine equilibrium constant of complex formation by thermometric titration. Equilibrium constant of complex formation reaction may be estimated from enthalpograms when there is distinct curvature near the equivalence point as shown in (where is Figure) Let us consider the reaction.
A + B ⇔ AB
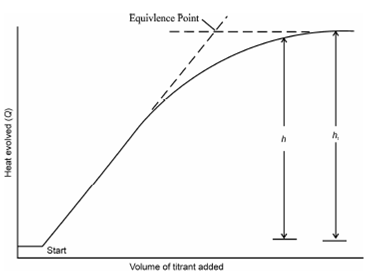
Figure: Equilibrium curvature of enthalpograms, illustrating parameters h and ht used to estimate equilibrium constants
where equilibrium concentrations [A] and [B] can be calculated using analytical concentration (A) and (B). Thus we have
[AB] = h/ ht ( A)
[A] = (A) - [AB]
[B] = (B) - [AB]
These concentrations are then used to calculate quilibrium constant (K) using the expression
K = [AB]/ [A][B] = h( A)/ht [( A) - [AB][( B) - [AB]]]
Consider the reaction between a metal (M) with a ligand (L) to form a complex ML Initial concentrations for both are given to be 0.015 M. It gives an enthalpogram similar to Figure where h and ht were measured to be 58.2 and 67.9 (0ºC, cm, mV or in any other units) respectively wherefrom stability constant of the complex can be estimated as described in following lines.
At the end point, following relationship holds.
CL = 0.0015 = [ML] + [L]
and CM = 0.0015= [ML] + [M]
But [ML]=CM -{h/ht} = CL-{h/ht}
[L]=[M]=CM-[ML]=CL-[ML]
Thus stability constant may be calculated as
K =[ML]/ [M][L] = C M h/ht (CM - [ML])2= 0.015 × 58.2/67.9 [0.015 - 0.015(58.2 / 67.9)]2 = 2.8 × 10 3
When dilute solutions are used, it may be safely assumed that the measured thermodynamic parameters are essentially the same as in standard state of infinite dilution (?Hmean ≈ ?Hº).