Classical example of thermometric titration:
A classical example of thermometric titration is in the titration of boric acid (Ka = 6.4 × 10-10) with a strong base (such as NaOH) which is otherwise very difficult to perform.
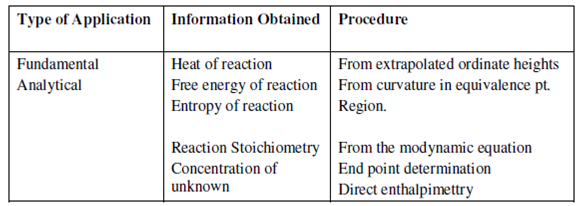
Table: Summary of Application of Thermometric Titrations
As an illustration, Figure shows comparision of potentiometric (pH) and thermometric titration curves for HCl and H3BO3 with NaOH in dilute aqueous solution. The potentiontiometric curves of the two acids are very different yielding a large end point inflexion 'for the HCl (Ka~ ∞) and virtually none for boric acid. Therefore, corresponding enthalpograms (are strikingl y similar, because the heats of neutralization of the two acids are comparable, -13.5 kcal/mole for HCI and -10.2 kcal/mole for H3BO3. As a result H3BO3 can be determined with better precision and accuracy by thermometric titration compared to potentiometric method. In the enthalpogram for H3BO3 and NaOH, AB represents a trace of the temperature of the solution before the addition of titrant and C is the end point. Line BC shows the gradual evolution of heat of reaction. Linear portions of the curves are extrapolated to give the initial and equivalence points and the vertical distance between them (BB´) gives temperature difference (?T) used to evaluate enthalpy.
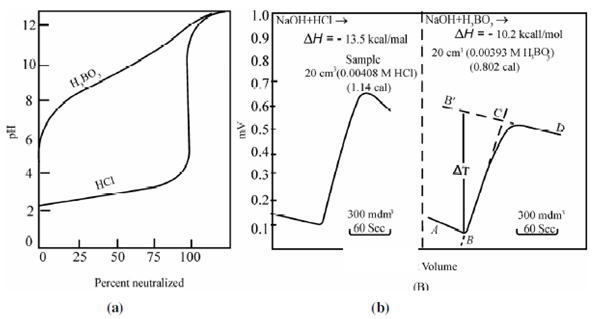
Figure: Comparision of (a) potentiometric and (b) thermometric titration curves for HCl and H3BO3 with NaOH solution