Free energy change
The change in Gibbs free energy ΔG dictates whether a reaction will be energetically favorable or not. In the Figure which is shows below an instance where the whole energy change of the reaction makes it energetically favorable example the products are at a lower energy level than the substrates and ΔG is negative. It should be noted that ΔG is not related to ΔG‡. The ΔG of a reaction is independent of the path of the reaction and it gives no information about the rate of a reaction since the rate of the reaction is governed through ΔG‡. A negative ΔG show in which the reaction is thermodynamically favorable in the direction indicated for example that it is likely to occur without an input of energy, while a positive ΔG indicates in which the reaction is not thermodynamically favorable and needs an input of energy to proceed in the direction indicated. In the biochemical systems this input of energy is frequently achieved through coupling the energetically unfavorable reaction with a more energetically favorable one coupled reactions.
It is frequently convenient to refer to ΔG under a standard set of conditions, described as when the substrates and products of a reaction are all present at focusing of 1.0 M and the reaction is taking place at a constant pH of 7.0. Under these conditions a slightly several value for ΔG is found, and this is known as ΔGo’. An instance of an energetically favorable reaction that has a large negative ΔGo’ and is generally used to drive less energetically favorable reactions is the
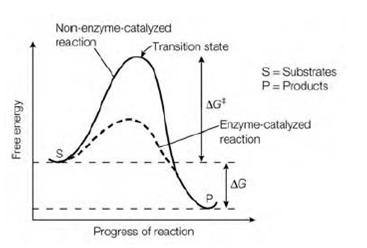
Figure: The energy changes taking place during the course of a biochemical reaction.
hydrolysis of adenosine triphosphate ATP; Figure to form adenosine diphosphate ADP and free inorganic phosphate (Pi):
