Chemical equilibria
A chemical reaction commonly exists in a state of dynamic equilibrium, where while new molecules of product and substrate are continually being formed and transformed , the ratio of substrate to product remains at a constant value.
Consider the reaction:
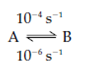
where the rate of the further reaction is 10-4 per second (s-1) and the rate of the reverse reaction is 10-6 s-1. By equilibrium the ratio of the concentrations of the substrate and product gives a constant value which is called as the equilibrium K constant. The equilibrium constant for a given reaction is shown as:
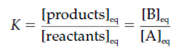
where square brackets shows concentration. Equilibrium constant is also given through the ratio of the forward reaction rate (kf) and the reverse reaction rate (kb):
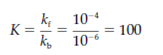
Therefore, for the above reaction at equilibrium, there is 100 times more product of B than there is of substrate A, apart from of whether there is enzyme present or not present. This is because enzymes do not alter the equilibrium position of a reaction,
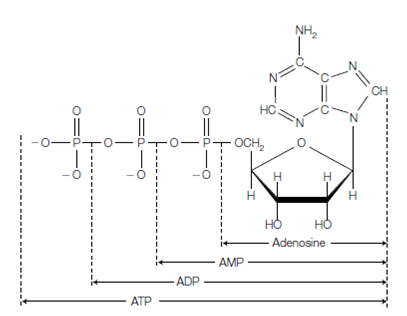
Figure: Structure of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP) and adenosine.
but accelerate the reverse and forward reactions to the similar extent. Alternatively, enzymes accelerate the attainment of the equilibrium position but do not shift its position. For the hypothetical reaction described above, in the absence of added enzyme the reaction may take over an hour to reach the equilibrium position, while in the presence of enzyme the equilibrium position may be reached in less than 1 s.