Specific Heat
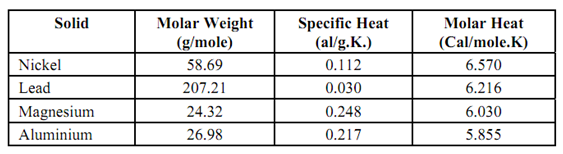
Specific heat is the amount of heat absorbed in the solid for 1 degree rise in temperature perunit mass. Dulong and Petit law (1819) states that molar specific heat for all elementary solid materials is same and its value has been shown to be 6 cal/mole. If, however, the specific heat is expressed as per gramme it will be different for different materials. Table will show the difference between molar specific heat and specific heat per g.
Table: Specific and Molar Heat at Room Temperature
Einstein and Debye had proposed that heat absorption of solid material is by way of enhancing in internal energy comprising kinetic energy of atoms (vibration) & kinetic energy of electrons. The former predominates. Deybe had proposed that vibration of atoms was affected by coupling effects among unit cells that make crystalline solids. They theoretically calculated the specific heat of solids showing variation on below zero degree C or close to absolute zero. They also establish that specific heat reaches a constant value in the range of most applications from room temperature. Diamond might be only solid whose specific heat increases continuously even up to 1000oC.