Raman Activity of Vibrations:
As a first response, the answer to the question about the similarity of IR and Raman spectra raised above, could be within a firm affirmative or one might respond, 'may be'. However in actual practice the two spectra are generally not identical and in case of a number of molecules, the spectra in fact do not have anything in common. This is so because the very mechanisms by which the two spectra arise are different. This feature actually is an important indicator about the symmetry of the molecule.
The Raman and IR spectra for a simple molecule CCl4 is given in Figure. You may note that only some of the signals are common in the two spectra and also the common signals are of different relative intensities.
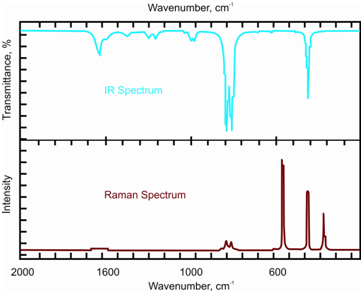
Figure: IR and Raman spectra of CCl4; note the similarities and the differences
You would recall from the previous unit on IR spectrometry that all the possible vibrational modes of a molecule may not be IR active. Only those modes which have an associated change in dipole moment are IR active and show up in the spectrum while the other modes are absent. As described above, in Raman spectrum only those vibrational modes are active which are associated with a change in polarisability. Due to this difference in the requirements for showing the two types of spectra, the IR and Raman activities of the vibration modes may differ from each other.