Types of Nuclei
You know from your earlier classes that the atomic nucleus is positively charged and some of the nuclei are associated with a fundamental property called spin. A nonspinning and spinning nucleus may be represented as shown in Figure. The angular momentum of the spinning nucleus is defined in terms of spin angular momentum quantum number, I. A magnitude of spin angular momentum |Ι| of the spinning nucleus is related to the spin angular momentum quantum number, as given below:
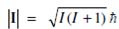
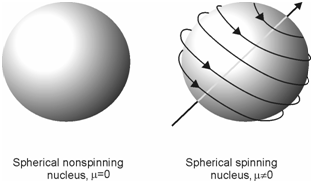
Figure: Illustration of non spinning (I = 0) and spinning (I = ½) nucleus
The spin angular momentum of a nucleus is vector sum of the spin angular momenta of the component particles of the nucleus, namely a neutrons and protons. An exact way within that the neutrons and protons are vectorially coupled can be understood from nuclear shell model. Therefore, at this stage it is sufficient to know about some generalisation for the spin quantum number of different nuclei:
i) Nuclei having even number of protons and neutrons have I = 0. The examples of such nuclei are 4He, 12C or 16O.
ii) Nuclei having odd number of protons and neutrons have integral value of I. For example, 2H and 14N have I = 1.
iii) Nuclei will having odd value for the sum of protons and neutrons have half integral value of I; for example, 1H and 15N have I =1/2 and 17O has I = 5/2 .
The nuclei with I = ½ are important for NMR; proton (1H) being probably the most exploited nuclei in NMR.