Nuclei other than Protons:
You would recall that only those nuclei show NMR that have nuclear spin angular momentum quantum number, I ≥ ½ . More than 200 isotopes in principle can be studied through NMR. Of these, 13C, 19F or 31P are most hugely used besides 1H. The characteristics of these nuclei are given in Table. In many organic compounds where proton magnetic resonance spectrum does not provide unambiguous information and carbon-13 NMR spectra have been found to be especially useful for structure elucidation. 13C NMR spectra are intrinsically much less sensitive than proton NMR spectrum. The low signal strength gives output from the fact in which the natural abundance of 13C is only 1.11% and also its magnetic moment is ¼ th of that of the proton in a given magnetic field.
Table: Characteristics of some nuclei commonly exploited in NMR
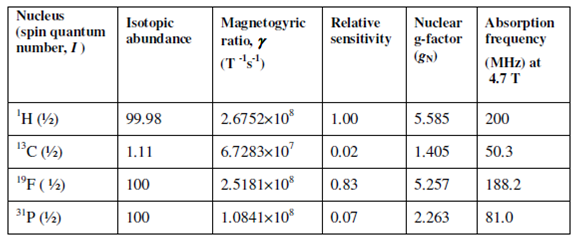
Besides 13C, two other most widely investigated nuclei are 19F and 31P, both of which not only have I = ½ but both of these nuclei have natural abundance of 100%. In these cases NMR spectra can be analysed in terms of their characteristic chemical shift (δ) and spin-spin coupling constants (J) due to their interaction with other nuclei. In addition to these, other nuclei like as 2D, 11B, 15N, 29S1, 23Na, 109Ag, 199Hg, 113Cd and 207Pb have also been investigated. NMR of those nuclei is particularly significant in the field of Organic Chemistry, Biochemistry, Biology, Organometallic Chemistry, alloys and intermetallic compounds.