Mechanism of Resonance
Experimentally, there are two different ways to achieve resonance. To understand these, let us have a look at the Eq. 12.5 again. The equation shows that there are two variable parameters: (i) frequency (υ) and (ii) magnetic field (B0); gN, µN and h being constants. One obvious way to achieve resonance is to vary the frequency at a fixed magnetic field strength. This technique is known as the frequency sweep method. In this method the sample is placed between the poles of a strong magnet which generates two energy levels and causes the nucleus to undergo precessional motion. The sample is then irradiated with a variable radiofrequency generated by an oscillator or transmitter. The resonance condition is achieved when the frequency of the radio frequency radiation matches with the Larmor precessional frequency. Under this condition known as the resonance, nuclei within the lower spin state absorb the radiation and go over to the higher energy state. This transition is commonly called as 'spin flip'.
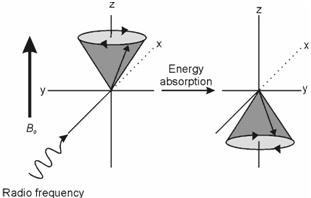
Figure: Spin flip: Absorption of the radio frequency radiation causes the α spin to change (flip) to β spin
Alternatively, we can keep the oscillator frequency constant and vary the magnetic field strength. This technique is known as the field sweep method. While the field strength is low the precessional frequency is lower than the applied radiofrequency and because the two frequencies do not match, no energy is absorbed. As we raise the field, the precessional frequency begins increasing and at a certain field strength it matches exactly with the applied radio frequency and the resonance condition is achieved and the radiation is absorbed. That causes the transition from α to β state. In the general instruments, both methods are used; however, the field-sweep method is preferred. It is because of relative ease of varying the field. In field sweep spectrometers the continuous variation of the magnetic field strength is achieved with the help of special coils present at the poles of the magnet.