Thawing And Freezing:
Consider our old pal, water. Visualize that it is late winter in a place like northern Wisconsin and that the temperature of the water ice on the lake is accurately 0°C. The ice is not safe to skate on, since it was in the middle of the winter, as the ice has become "soft." It is more like slush than ice. It is partially solid and partially liquid. However, the temperature of this soft ice is 0°C.
As the temperature continues to increase, the slush gets softer. It becomes proportionately more liquid water and less solid ice. Though, its temperature stays at 0°C. Ultimately, all the ice melts into liquid. This can take place with amazing rapidity. You may leave for school one morning and see the lake closely "socked in" with slush and return in the evening to find it almost wholly thawed. Now you can get the canoe out! Though you won't desire to go swimming. The liquid water will remain at 0°C until all the ice is gone. Only then will the temperature start to rise slowly.
Consider now what occurs in the late autumn. The weather, and water, is growing colder. The water ultimately drops to 0°C. The surface starts to freeze. The temperature of this new ice is 0°C. Freezing occurs until the entire lake surface is solid ice. The weather remains growing colder (a lot colder when you live in northern Wisconsin). Once the surface is totally solid ice, the temperature of the ice starts to fall below 0°C, though it stays at 0°C at the boundary just under the surface where solid ice meets liquid water. The layer of ice acquires thicker. The ice near the surface can acquire much colder than 0°C. How much colder based on different factors, like the severity of the winter and the quantity of snow that occurs to fall on top of the ice and lag it against the bitter chill of the air.
The temperature of water does not obey accurately along with the air temperature whenever heating or cooling occurs in the vicinity of 0°C. Rather, the water temperature obeys a curve something like that which is shown in figure below. In portion a, the air temperature is getting warmer; in portion b, it is getting colder. The water "stalls" as it thaws or freezes. The other substances exhibit this similar property whenever they thaw or freeze.
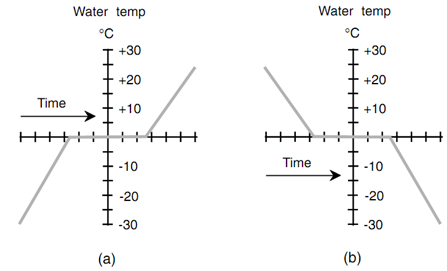
Figure: Water as it thaws & freezes. (a) The environmental temperature is getting hotter, and the ice is thawing. (b) The environmental temperature is acquiring colder, and liquid water freezes.