The International Temperature Scale
The International Temperature Scale (ITS) is an internationally decided practical standard scale. It is stated by identifying a number of fixed points (i.e., standard systems) altogether with a technique of interpolating among them (i.e., standard thermometers). The ITS determines a sequence of operational definitions for temperature in several ranges.
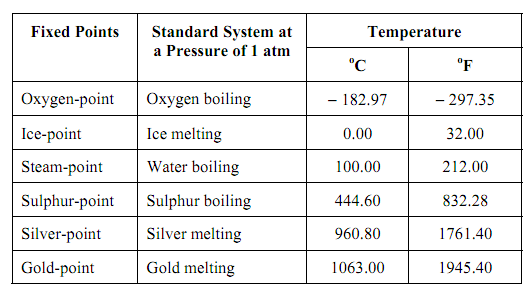
The table illustrates the fundamental fixed points that have been internationally approved. (A few additional secondary set points have also been stated).The numbers allocated to the different fixed points of the ITS have been selected in order that this scale must be steady with the absolute temperature scale, that is self-governing of the properties of specific substances or systems.
In order to identify temperatures among the fundamental fixed points, an interpolation process is arranged. The range of the scale is separated into four parts, and interpolation process is arranged. The range of the scale is separated into four parts, and for each part a specific kind of thermometer is identified as the indicator of the temperature. For illustration, among the ice-point and 660oC, the temperature t is deduced from the resistance Rt of a standard platinum resistance thermometer, via the equation as follows:
Rt = R0 (l + At + Bt2)
The constants R0, A and B of this equation are established by calibrating the thermometer at the ice, steam and sulphur points. Various means of interpolation are identified for the other three parts of the scale. Most of the information on the ITS is available from standard references like the Handbook of Physics and Chemistry.
The organization of the ITS makes it likely for scientists and technologists to agree on temperature measurements. Therefore the practical problems formed by the randomness of temperature scales have been resolved by the agreement on the ITS. The hypothetical problems, though, still remain. Temperature still appears to arbitrary. It can be place on a sound basis merely with the help of the second law.
A partial answer to the theoretical problem is provided by a kind of thermometer that is independent of the physical properties of the substances or materials employed in building the thermometer. It is the ideal gas thermometer. Additionally, it gives readings that are quite similar with those on the absolute scale.