The Ideal Gas Thermometer
The ideal gas thermometer includes an extrapolation of answers to zero pressure at which all gases behave as ideal gases. The figure shown below illustrates a gas thermometer. It consists necessarily of a glass bulb associated to a U-tube having liquid like, for illustration, mercury. A “permanent” gas (example, nitrogen, oxygen, hydrogen) is surrounded within the bulb and the joining flexible tube by the mercury, the other limb of the U-tube being open to the environment. Throughout the experiments, the bulb is located in contact with systems at various temperatures, and the level of the mercury can be accustomed to deep either the volume or the pressure of the gas constant
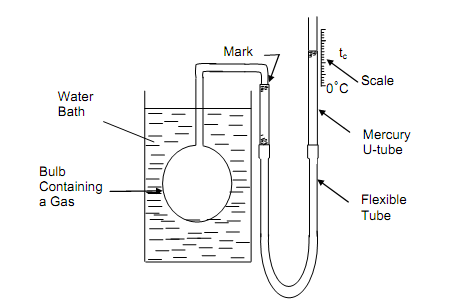
Figure: Constant-Volume Gas Thermometers
Let consider operation as constant-volume gas thermometer. In order to set up the temperature scale, we note down the difference in the right-hand meniscus level (i.e., indicative of the gas pressure) at the two set points, keeping the mercury level in the left-hand limb, therefore the gas volume, constant in each situation. A gas thermometer Celsius scale is stated by assigning 0 and 100 to the ice and steam points, with 100 equivalent sub-divisions among them. Such linear scale in gas pressure p may be stated as follows:
pS = pi (1 + α t)
Here:
pi = The gas pressure at the ice point, 0oC,
t = The “gas thermometer” Celsius temperature,
α = A constant, equivalent to (ps - pi)/ 100 pi, and
ps = The gas pressure at the steam point, 100oC.
A sequence of values of α is acquired by performing the experiments using equivalent volumes of the same gas at gradually lower gas pressures (that is, smaller masses of gas).By plotting such values of α against pi in each experiment, and extrapolating the curve to zero pressure, a value of α equivalent to zero pressure might be established. In practice, α differs little with pressure.
In a similar manner, by the use of a number of various gases, a value of α equivalent to zero pressure is acquired for each gas. The significant experimental outcome is that such values of α confirm to be similar for every gas. The behavior of gases at very low extrapolation states the ideal-gas and the value of α so acquired by extrapolation states the ideal gas temperature scale. The ideal gas Celsius scale is specified by :
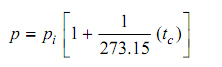
It can be observed from the above, the extrapolated value of α equivalent to zero pressure is 1/273.15. A corresponding equation, with similar value of α, though with volume substituting pressure, is acquired by using the constant-pressure gas thermometer.
It can be observed from the equation that whenever the pressure p is zero, t = − 273.15oC on the ideal gas Celsius scale.
In addition to give us with a more acceptable idea of temperature, the Kelvin (and Rankine) scales point out a very significant feature: only positive absolute temperatures are stated. Negative Kelvin (or Rankine) temperatures do not survive. It is for this purpose that such scales are termed as absolute. As a matter of fact the zero point on such scales is termed as the absolute zero to differentiate it from the zero points on the Fahrenheit (and Celsius) scales.