Temperature Scales
Temperature scales are stated by assigning numbers to the ice point, to the steam point, and to the number of partitions into which the interval is separated. The Celsius temperature scale has the ice point marked as 0, while the steam point marked as 100, with 100 equivalent sub-divisions. Now, when a thermometer S3 graduated in this way, is brought into communication with the system S1, and the mercury rises to the sub-division marked 60, we say that the system S1 has a temperature of 60 degrees Celsius, or written as 60oC. The size of each of the 100 sub-divisions states the unit of temperature, Kelvin (K).
The other temperature scale that prevailed till lately (and yet in vogue in North America) is the Fahrenheit scale, in which the ice point and steam point are marked as 32 & 212, correspondingly, with 180 equivalent intermediate intervals. The size of the unit for such scale is the Rankine (R). The relationship among the Celsius and Fahrenheit temperatures of a specified body, tC and tF is:
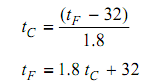
It is informative to trace the history of the temperature scales; the general measure of temperature in terms of degrees Fahrenheit is a result of Sumerian of Babylonian persuade; minutes, seconds, and degrees. During the early portion of the eighteenth century, the Danish astronomer Ole Romer selects the boiling point of water as his high fixed point and the temperature of a mixture of ice, salt & water as the lower fixed point. Following the Babylonian tradition he accepted sixty divisions for his thermometer, and hence his lowest and highest temperatures became 0 and 60, correspondingly. Romer observed that water froze at around 7.5 or 8 degrees, and that common body temperature was around 22.5 degrees, on his thermometer.
In 1708 Gabriel Daniel Fahrenheit tripped Romer in Copenhagen and this led him to accept the same fundamental principles in his own work, with some minor modifications. Fahrenheit select the similar lower fixed point though took the body temperature as his upper fixed point. He found Romer’s scale to be so coarse; therefore he partitioned each of the 60 subdivisions into four portions. The freezing point of water therefore became 4 × 8 = 32, and the body temperature 4 × 22.5 = 90.
Afterward he changed the scale a little and hence the normal body temperature would communicate to 96 rather than 90; on this altered scale he found that water boiled at 212 instead of at 240, therefore resultant in 180 degrees among the freezing and boiling points of water. The Fahrenheit thermometers were consequently calibrated on the bases of the freezing and boiling points of water, and hence Fahrenheit’s original upper fixed point, namely, the body temperature, turned out to be only an incidental intermediate point at 98.6.
The origin of the thermometer scale with 100 subdivisions among the boiling and freezing points of water are not so apparent. The first proposal emerges to have been made by Elvius of Sweden in the year 1710. The centigrade system is, though, credited to Anders Celsius, a Swedish astronomer, who used the centesimal system in the year 1742. Celsius fixed the boiling point of water at 0 and the freezing point at 100. It is supposed that he did this to avoid negative temperatures beneath the melting point of ice. (It is not recognized how he was going to attempt negative temperatures above the boiling point of water). Few years later, one of his colleagues, Marten Stromer overturned the fixed points and generated the centesimal scale we use nowadays. It is no longer termed to as the “centigrade” scale, though as the “Celsius” scale.
After knowing the second law of thermodynamics, the “absolute” scale of temperature will be introduced and its importance will be described. This scale was proposed in the year 1851 by Lord Kelvin and is named after him; it puts the absolute zero of temperature at 273.15oC below the freezing point of water at atmospheric pressure. The absolute scale equivalent to the Fahrenheit temperatures is termed as the Rankine scale, and positions the absolute zero at 491.67oF below the freezing point of water.
As far as the number of set points is anxious, Guillaume Amontons, a French meteorologist, indicated in the year 1701 that only one fixed point is really essential. (Incidentally, he was also the primary one to imagine of an absolute scale of temperature). This suggestion was reinforced by Lord Kelvin, and again revitalized by W. F. Giauque of the University of California in the year 1939. It has been consequently accepted in the triple point of water, the grouping of pressure and temperature at which all the three phases of water (i.e., solid, liquid & vapor) coexists in symmetry. For water, the triple point temperature is allocated the value is selected to sustain the traditional difference of 100.00 K among the boiling point of water (i.e., 373.15K) and the melting point of ice (that is of 273.15 K).