Extrapolation Beyond The Fixed Points
How can we evaluate temperatures below and above those of the two set points? The uniform graduations among the fixed points might also be continued below and above the fixed points. If, for illustration, the mercury increases 40 divisions above the steam point mark of a Celsius thermometer, the temperature is then considered to be 140oC.
This way of extrapolation is limited in practice by material properties: When the temperature falls too low, mercury freezes (at – 38.9o), whereas at high temperatures glass becomes an inappropriate casing material. The strategies for overcoming such constraints involve the use of various fluids and casing material, or by using dissimilar thermometric property.
Every thermometer has a thermometric property related with it. The Zeroth law makes sure that the reading of the thermometer is the temperature of all systems in thermal symmetry with it. The properties below are desirable in a thermometer:
Sensitivity: A considerable change in the thermometric property generated by an associative small change in temperature.
Accuracy: Readings close to agreed-upon the standards.
Reproducibility: Readings that can be duplicated constantly.
Response: Quickly coming into the thermal equilibrium with the system whose temperature is being measured.
Apart from volumetric expansion, some other thermometric properties in fashion are as follows:
(a) Electrical resistance of the platinum wire.
(b) Potential difference among the junctions of different metals (like thermocouple).
(c) Thermal (i.e., infra-red) or visible radiation (i.e., pyrometers).
For measuring temperatures beneath the freezing point of mercury, ethyl alcohol (i.e., ethanol) in glass can be employed down to temperatures of – 110oC. Though, such a change to new thermometric substances introduces a trouble. An alcohol-in-glass thermometer and a mercury-in-glass thermometer, both measuring the temperature of the similar system, give, in common, dissimilar readings, even although they were graduated with reference to the similar standard systems. The figure shown below indicates this behavior, the difference being shown exaggerated for illustration. Both the scales should agree at the fixed points, by definition. It is seen that these are, however, the only two points at which the two scales concur. In the illustration shown, when tm is 60oC, ta is 59oC. The query is: which thermometer is to be taken as accurate?
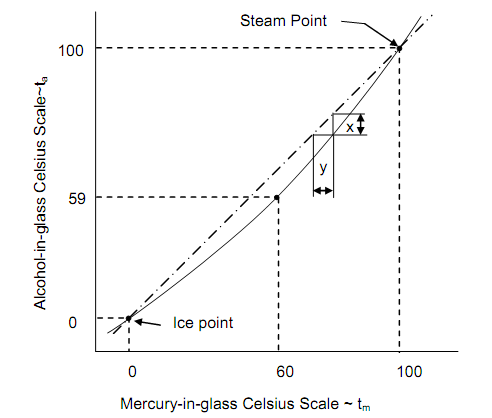
Figure: Comparison of Temperature Scales
The similar problem occurs also whenever other thermometric properties are used over the similar range. Apart from at the calibration points, such thermometers will not usually agree. The reason is that each of the thermometers based on how each different physical property – the the electromotive force of a thermocouple, volume of mercury, the electrical resistance of a wire, and so on. – differs with temperature. There is no reason why all such physical properties of different real materials must be similar function of temperature; however, they are not.