Algebraic Sign Convention
The heat interaction is generally specified by the symbol Q, and is considered to be positive for a system when the system is at lower temperature in comparison to its environment. It means that heat transfer to the system is positive, whereas heat transfer from the system is negative. The effect of this convention is that when, in a given communication, the heat for the system is positive that for the surroundings is negative.
It will be observed that this convention is just the reverse to that for work: as is shown in figure below, where the arrows state symbolically the “transfer” metaphor.
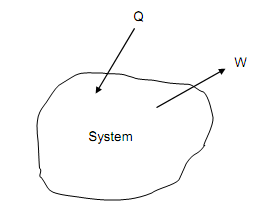
Figure: Sign Convention for Heat and Work
There is a historical purpose for such conventions. At the time the ideas of heat and work, and their inter-conversion, were primary being studied, the principal application region was the operation of the steam engines that were essential for pumping out the water from the coal mines, that incidentally given the fuel for the steam engines. With efficiency stated as the ratio of output to input, the output being the work conveyed by the steam engines, and the input being the heat conveyed to the working fluid, it was essential to use the sign convention in order to come up with positive effectiveness values.
At times the directional language and the sign convention might appear concurrently in phrases like “the heat transfer to the system is negative”, pointing that the direction of heat transfer is from the system to its surroundings.