Temperature Scales
The two temperature scales generally employed for measurement aims are the Fahrenheit (F) and Celsius (C) scales. Such scales are based on a specification of the number of increments among the freezing point and boiling point of water at standard atmospheric pressure. The Celsius scale has 100 units among such points, and the Fahrenheit scale contains 180 units. The zero points on the scales are random.
The freezing point of water was chosen as the zero point of the Celsius scale. The coldest temperature attainable with a mixture of ice and salt water was chosen as the zero point of the Fahrenheit scale. The temperature at which the water boils was put at 100 on the Celsius scale and 212 on the Fahrenheit scale. The connection among the scales is symbolized by the equations below.
°F = 32.0 + (9/5)°C
°C = (°F - 32.0)(5/9)
It is essential to define an absolute temperature scale containing only positive values. The absolute temperature scale which corresponds to the Celsius scale is termed as the Kelvin (K) scale, and the absolute scale which corresponds to the Fahrenheit scale is termed as the Rankine (R) scale. The zero points on both absolute scales symbolize the similar physical state. Such state is where there is no molecular motion of individual atoms. The associations among the absolute and relative temperature scales are shown in the equations below.
°R = °F + 460
°K = °C + 273
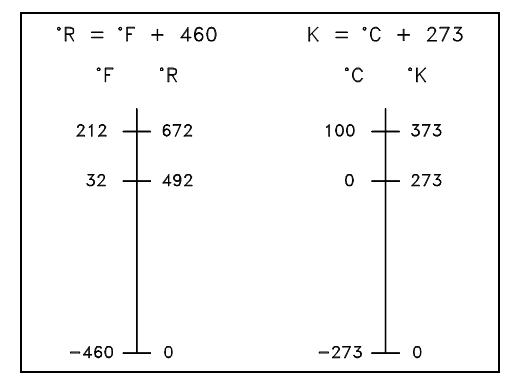
Figure: Comparison of Temperature Scales
The conversion of one temperature scale to the other is at times needed at nuclear facilities, and the operator must be familiar with the procedure.
Example:
Determine the Rankine equivalent of 80°C?
Solution:
°F = (9/5) °C + 32
= (9/5)(80) + 32
= 176 °F
°R = °F + 460
= 176 + 460
= 636 °R