Extraction of Metal Chelates:
In the classification of extraction systems under extraction by compound formation, it has been described in which the chelating agents form a very important class of extractants. A Metal chelates represent a type of coordination compounds in which the metal ion combines with a polyfunctional base, capable of occupying two or more positions of the coordination sphere of the metal ion, to form a cyclic compound.
A large number of chelating agents are used for metal ion extractions. It may be a big task even to list a few important ones primarily because of their availability in large number and variety. But as an instance, we might consider the reagent 8-quinolinol (8- hydroxyquinoline) which is often referred to by the trivial name "oxine".
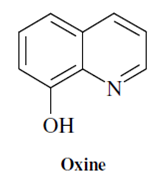
This organic compound forms neutral species with a number of metal ions which is insoluble in water but soluble in chloroform or carbon tetrachloride. If oxine is abbreviated HOx, the equilibria can be written as
Mn+ + n HOx ↔ M (Ox) n + n H+