Synthetic planning:
Knowledge of the functional group and electrophilic substitutions transformations that are possible is necessary in planning the synthesis of an aromatic compound. While designing such type of a synthesis, it is good to work backwards from the product and to ask what it could have been synthesized from - a process termed as retro synthesis. We can demonstrate this through designing a synthesis of an aromatic ester. An ester functional group cannot be linked directly through electrophilic substitution, thus the synthesis must involve various steps. The general way to make an ester is from an acid chloride that is synthesized in turn from a carboxylic acid. Otherwise, the ester can be made directly from the carboxylic acid through treating it with an alcohol and an acid catalyst. Either way, benzoic acid is needed to synthesize the ester. Carboxylic acids cannot be added straight to aromatic rings either, thus we have to look for a dissimilar functional group that can be added directly, after that transformed to a carboxylic acid. A carboxylic acid group can be acquired from the oxidation of a methyl group. Methyl groups can be added directly through Friedel-Crafts alkylation. Hence a possible synthetic route would be as displayed in figure.
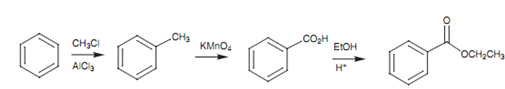
Figure: Possible synthesis of an aromatic ester.
One probable problem with this route is the possibility of poly-methylation in the 1st step. This is likely because the product (toluene) will be more reactive as compared to the starting material (benzene). One way round this problem would be to make use of an excess of benzene.