Synthesis of m-toluidine:
The synthesis of m-toluidine needs a little much more thought. Both of the methyl and the amino substituents are activating groups and direct ortho/para. Though, the two substituents are meta with respect to each other. To acquire Meta substitution we need to introduce a substituent other than the methyl or nitro group that will direct the second substitution to the meta position. Furthermore, once that has been acquired, the Meta directing substituent has to be transformed to one of the needed substituents. The nitro group is ideal for this because it directs Meta and can then be transformed to the needed amino group.
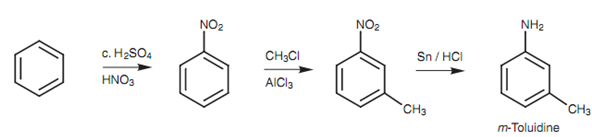
Figure: Synthesis of m-toluidine.
This similar strategy can be employed for a large range of meta-disubstituted aromatic rings in which both substituents are ortho/para directing because the nitro group can be transformed to an amino group that can then be transformed to a large range of dissimilar functional groups. Other tricky situation is where there are two meta-directing substituents at ortho or para positions with respect to each other, for instance, p-nitrobenzoic acid. In this case, a methyl substituent is added that is o/p directing. After that the nitration is carried out and the para isomer is separated from any ortho isomer that might be formed. After that the methyl group can be oxidized to the needed carboxylic acid.
Larger alkyl groups could be employed to raise the ratio of para to ortho substitution because they can all be oxidized down to the carboxylic acid.
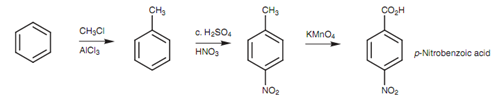
Figure: Synthesis of para-nitrobenzoic acid.